Source
Arabian Journal of Geosciences, (2016)
Abstract
The purpose of this research is to evaluate the groundwater quality in Dindugal district of Tamil Nadu based on the water quality index by geographic information system(GIS) and statistical analysis. This area consists of 80 functional tanneries around Dindigul town with a capacity to process about 200 Mt of hides and skins as leather. In 13 villages,as many as 1090 houses were damaged by tannery contamination. A total of 66 groundwater samples were collected to identify the geochemical sources and contamination. The order of major cations is Na > Ca > Mg > K, while that of anions is Cl > SO4 > HCO3 > F > PO4 . CaCl2 , MgCl2 , and (CaHCO3)2 types suggested that the mixing of high-salinity water was caused by irrigation return flow, domestic wastewater, and septic tank effluents, with existing water followed by ion exchange reactions. Moreover, Gibbs plots indicated that groundwater contamination was derived from the weathering of granitic gneisses as well as the leaching of evaporated and crystallized ions from agricultural and industrial effluents.
The water quality index (WQI) exhibited 8% of the groundwater samples were not suitable for drinking purpose. The GIS maps showed that the poor water quality decreased toward the southern part of the study area. WQI of TDS, fluoride, sodium, potassium, and bicarbonate were high in groundwater. Multivariate statistical analyses (principal component analysis (PCA), factor analysis (FA)) suggested that the groundwater chemistry was changed by the weathering of source rocks ion exchange and leaching ofinorganic components and addition from anthropogenic effluents. Finally, it is thought that the monitoring and assessment works are very useful to understand the degree and sources of groundwater contamination.
Keywords Groundwater contamination. Gibbs plot. WQI. GIS. Multivariate statistical analyses
Introduction
The natural resources of groundwater are utilized by drinking, irrigation, and industrial purposes. Groundwater quality and quantity are heterogeneously distributed in space and always vary in time (Singaraja et al. 2015; Venkatramanan et al. 2012, 2015; Vasanthavigar et al. 2012). These groundwater resources are sustained only when quantity and quality ofgroundwater are properly assessed. Nowadays, the population is gradually increased at a certain rate because of growth and the usage of groundwater is also increased rapidly. Due to such overexploitation of groundwater, resources are contaminated by various man-made activities. In many parts of India, especially in the arid and semi-arid regions, due to impulse of monsoon and shortage of surface water, assurance on groundwater resources is enlarged extremely in the past few years.
It is viewed in the international perspective of B3/person/ year^ as water stressed and B3/person/year^ as water scarce. Future groundwater resource status of India is limited in 2050 due to groundwater deterioration (Antony Ravindran and Selvam 2014; Selvam et al. 2015a,b, c; Srinivasamoorthy et al. 2011; Venkatramanan et al. 2014). Groundwater quality is equally important, as its quantity owed to the suitability of water for various purposes. The gap of groundwater quality in an area is a function of physical and chemical parameters that are significantly influenced by natural and anthropogenic activities. The excellence of world water resources is being progressively degraded as an importance of its intensified anthropogenic management.
Tanning industry is one of the oldest and fastest growing industries in South and Southeast Asia. About 88%of tannery industries are present invarious states of India, such as Tamil Nadu, West Bengal, and Uttar Pradesh. Nearly 55 % of the total leather processed in India is from Tamil Nadu, and its tannery units are spread overPallavaram and Chrompet in Chennai, Ranipet, Ambur, Vaniyambadi, Pernambut of Vellore, Dindigul district, parts of Erode district, and Sembattu in Trichy district.
There are about 80 functional tanneries around Dindigul town with a capacity to process about 200 Mt of hides and skins as leather. It was estimated that about 76,400–85,600 kgof leather was produced in Dindigul town every day (Magesh and Chandrasekar 2013; Paul Basker, 2000). Most of the tanneries are present along the roads of Madurai, Batla Gundu,and Ponmandurai. This industry was commenced in the year of 1939, and the process of tanning involved the use of large amount of freshwater and various chemicals. The variouschemicals that were used in tanning were lime, sodium carbonate, sodium bicarbonate, common salt, sodium sulfate, chrome sulfate, fat, liquors, vegetable oils, dyes, etc. Thisindustry is one of the major consumers of freshwater, and most of the water is discharged as wastewater.
A total of 100 kg wastewater was discharged for skin and hide processes, whichranged from 3000 to 3200 l. Common salt (NaCl) is the biggest polluting material in the tanning industry. For every 10 T of salted hides and skins processed, 2–3 T of salt are removed and, in addition, another 1 Tof salt is detached, while pickling. Tannery industries generated the amount of wastewater approximately ranging from 2.5 to 3.0 million liters per day (MLD), with total dissolved solid (TDS) level of 20,000–25, 000 mg/l on the surface, which, in turn, is collected in irrigation ponds (Mondal et al. 2005; Selvam2014a and b).
A groundwater quality map is most important for drinking and irrigation purposes and as preventive warning of potential environmental health problems. Dindugal district is muchmore difficult because of spatial variability for multiple contaminants and wide range of indicators that could be measured. There were many publications associated with identification of groundwater potential zones from resistivity surveys and remote sensing data, but no systematic updated publication/database was available for a water quality index(WQI) map for this study region. The current research work of this district will provide an updated scientific basis and may offer valuable vision for future research. The main researchgoals of this present work evaluate to identify the sources and contamination of groundwater used by geographic information system (GIS) and statistical interpretation in acomprehensive and integrated approach. Therefore, this research will assist as a base to access the enhancement of groundwater quality in the future.
Research site description
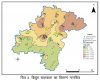
Dindigul region is composed of a hard rock terrain in Tamil Nadu, South India, and it lies between 10.35° N latitude and 77.98° E longitude (Fig.1a). The average rainfall and temperature of this region are 700 mm and 26∼38 °C, respectively. The main rivers in this district are Dodaganaru, Palar, Kuthiraiyar, Porandalar, Amravati, Manjalar, Varadhamanathi, and Maruthanathi. Three different climatic conditions prevail in this district. Tropical and sub-tropical climate are prevalent in plains and in the Palani and Sirumali hills. Due to the favorable climate, all kinds of horticultural crops are cultivated in this district. A semi and tropical monsoon type of rainfall is predominant in this district.
The geology of this study area is composed of hornblende biotite gneiss (HBG) and charnockite (Fig. 1b). HBG is the dominant one, and charnockite is covered by hilly terrain inthe southern part. Moreover, two anorthosite patches are observed in the central and eastern margins. Some of granulite patches and garnetiferous quartzofeldspathic gneiss are also present. Other minor rock types are pyroxene granulite, pink augen gneiss, garnetiferous sillimanite graphite gneiss, and granite. The soil types are red loam, laterite soil, black soil, sandy coastal alluvium, and red sandy soil. Red loams are widespread in Dindugal district, except Kodaikanal, while red sandy soils are present in Nilakottai, Oddanchatram, and Palani. Laterite and black soils are dominant in Oddanchatram, Natham and Nilakottai, Oddanchatram, Palani, and Vedasandur, respectively.
Aquifer systems of this district are divided into (i) fissured, fractured, and weathered crystalline formations consisting of charnockites and granite gneisses and (ii) valley fill sediments (unconsolidated sediments) comprising clay, sand, silt, and kankar. Groundwater occurred in weathered and fractured crystalline formations with semi-confined to confined condition. The depth of weathering varied from place to place with less than 40 m depth. The number of saturated fracture zones ranged from 1 to 6 by 10∼164 m depth. Exploration condition of groundwater in deeper aquifer divulges that 11 % of wells yield more than 3 lps, whereas 15 % of wells varied from 1 to 3 lps. The yield of open wells in the district tapping form a weathered mantle of crystalline rocks ranging from 100 to 400 lpm for 2∼4.5 m drawdown. The dug and deep wells can withstand pumping of 3∼4 and 6∼8 h/day, respectively. In the case of valley, sediments were distributed along the valley portions ranging from 35 to 40 m depth in Natham and Sanarpatti blocks. They are characterized by deeper water levels with high fluctuation condition. Dug well types are the major groundwater extraction method of these zones. The wells can yield about 200 m3/day and sustain pumping of 3∼4 h/day (PWD, 2000).

Material and methods
Groundwater sampling and analysis
A total of 66 groundwater samples were collected in 2014 (Table 1). Each sample was collected in acid-washed polyethylene HDPE bottles pre-washed with dilute HCL. The bottlewas completely prevented from air bubbles. Various parameters like pH, total dissolved solid (TDS), and electrical conductivity (EC) are measured in the field using portable metersSystronics Digital pH Meter and ELICO Conductivity Meter (PE-138) (Table 1). After the collection, the bottles was tightly closed and labeled. Then, the samples were stored at 4 °C. Further analyzed were the parameters of groundwater, like calcium (Ca), magnesium (Mg), sodium (Na), and potassium (K), and major anions, such as bicarbonate (HCO3), carbonate (CO3), chloride (Cl), nitrate (NO3), sulfate (SO4), fluoride (F), total hardness (TH) and phosphate (PO4), in the laboratory using the standard methods given by the American Public Health Association (APHA 1995).
Total hardness was determined by EDTA titrimetric method using Erichrome Black T as the indicator (Selvam 2015). Sodium and potassium were estimated by using the Flame photometer model 360, and the argentometric method (Singaraja et al. 2015) was employed for the determination of chloride. Sulfate concentrations of the sample were determined by nephelometric technique (Hem 1991; Karanth 1991; Mondal et al. 2010; Selvamet al.2013a,b; Venkatramanan et al. 2013). Fluoride concentration in the water sample was determined by the SPADNS method (Venkatramanan et al. 2015).
The base map was prepared by Survey of India topographic sheets (58F and 58J) and digitized using the ArcGIS 10.1 software (Fig. 1a). GPSMAP® 64s handheld GPS (Garmin,USA) was used to find the sampling location, and coordinates were imported to the GIS software for spatial analysis. Inverse distance weighted (IDW) interpolation spatial analysis was used to create different thematic layers for each ions (Selvam 2012). This interpolated data exhibit spatially or estimate values between measurements. These values assessedfrom IDW interpolation are a weighted average of the surrounding sample locations and the weighted values calculated by inverse of the distance method (Burrough and McDonnell1998; Magesh et al. 2011: Selvam et al. 2014a, d). The mathematical and statistical results have been commutated from statistical package for social sciences (SPSS Ver.17) software.
Water quality index procedure
WQI provided a complete picture of surface and/or groundwater uses for human consumption. pH, EC, TDS, TH, alkalinity, calcium, magnesium, sodium, potassium,chloride, sulfate, nitrate, fluorides, and iron were used to calculate the WQI. These methods were divided into three steps such that (i) each chemical parameter was assigned a weight(Wi) based on their observed effects on primary health/their relative importance in the overall quality of water for drinking purposes.
A maximum weight value of 5 was allocated to theparameters due to the major effects on water quality and their importance in quality (viz., NO3, F, and TDS), while a minimum weight value of 2 was given to the parameters which are considered as not harmful (Ca, Mg, K). (ii) The relativeweight (Wi) of each parameter used as present weight (Wi) is computed, and relative weight (Wi) values for each parameter are calculated. (iii) A quality rating scale (qi) for eachparameter and each water sample was computed by the standard of BIS (1998), and the results were multiplied by 100 (Table 2). Finally, for computing the WQI, the water qualitysub-index (SIi) for each chemical parameter was determined (Ravikumar et al. 2013):
 1. Excellent: (WQI values 2. Good: (WQI values 51–100)
1. Excellent: (WQI values 2. Good: (WQI values 51–100)3. Poor: (WQI values 101–200)
4. Very poor: (WQI values 201–300).
5. Unsuitable: (WQI values >301)
Statistical analysis
Multivariate statistical analyses were performed by major ions and EC, pH and TDSs. This method was used to reduce and organize large hydrochemical datasets into groups with similar characteristics. The basic purpose of this analysis was to interpret the relationship of variables. The main advantage of principal component analysis (PCA) is that it identifying patterns by compressing the data by reducing the numbers of dimensions without much loss of information (Irawan et al. 2009; Kazi et al. 2009; Selvam et al. 2014b, c, e). It is designedto convert the original variables into new, uncorrelated variables (axes) called the principal components, which are linear combinations of the original variables (Sarbu and Pop,2005). This is a way to identifying and expressing data similarities and differences of variables.
Table 1 Statistical measures such as minimum, maximum, average, and standard deviation in the study area
 Factor analysis (FA) is one of the most significant components contributing to the data structure and the interrelationships between the variables (Lall and Sharma, 1996). It wasused to identify the sources of groundwater contamination by natural chemical weathering and other anthropogenic impacts. The correlation matrix exhibits an array of correlation coefficients between pairs of variables. Then, the matrix was diagonalized and its principal components (eigenvectors) were obtained. This is called as factor I, which is associatedwith the largest eigenvalue and is able to clarify the greatest amount of variance in the data structure. The second factor is indicated as uncorrelated to the remaining variance with thefirst factor.
Factor analysis (FA) is one of the most significant components contributing to the data structure and the interrelationships between the variables (Lall and Sharma, 1996). It wasused to identify the sources of groundwater contamination by natural chemical weathering and other anthropogenic impacts. The correlation matrix exhibits an array of correlation coefficients between pairs of variables. Then, the matrix was diagonalized and its principal components (eigenvectors) were obtained. This is called as factor I, which is associatedwith the largest eigenvalue and is able to clarify the greatest amount of variance in the data structure. The second factor is indicated as uncorrelated to the remaining variance with thefirst factor.



samples are within the permissible limit according to the WHO 2004 standards, shown in Table 1. EC helps to find the suitability of water for irrigation purposes. The EC valuevaried from 160 to 6250 μS/cm in the study area. Forty-eight percent of the samples belong to the desirable limit, while 52 % crossed the permissible limits, as shown in Table 3. Todetermine the suitability of groundwater for any purposes, it is essential to classify the groundwater depending upon their hydrochemical properties based on their EC values (Handa, 1969), which are represented in Table 3. Figure 2a clearly shows that a not permissible (NP) limit of EC was observed in N-NW, central portion, and some small part in southern parts. Rather, higher EC values were documented in the northern part due to the anthropogenic activities like waste disposal, sewage inflow, and agricultural runoff (Pandit 2002; Chidambaram et al. 2007; Selvam, 2014a; Selvam et al. 2013c). The southeast and southwest parts are considered as permissible limits.
TDS ranged from 88 to 3614 mg/l, with an average value of 930 mg/l. Twenty-seven percent of the groundwater samples are reflected as desirable for drinking purposes, and 40 % ofthe samples are represented as permissible for drinking while 30 % of the samples are useful for agricultural activities. The rest of the samples are considered as unfit for drinking and irrigation based on the report by Davis and DeWiest (1966) and Freeze and Cherry (1979) in Table 3. In relation to the WHO standards, 12 % of the samples exceeded the permissible limit and 88 % of the samples are within the permissible limit (Table 3). Higher values of TDS can be attributed to the contribution of ions from source rocks and, further, to higher residence time of groundwater in contact with the aquifer system. Moreover, the TDS value for drinking water should be less than 500 mg/l; only 27 % of the samples are suitable for drinking purposes.
The spatial distribution of TDS concentration is shown in Fig. 2b. According to theWHO standards, the maximum allowable limit of TH is 600 mg/l and the most desirable limit is 300 mg/l for drinking purposes. Table 3 shows the classification of groundwater based on hardness, which states that 5 % of the groundwater samples are considered as soft water and 25 % of the samples are indicated as moderately high. Then, 65 % of the groundwater samples are measured under hard water and the remaining 5 % of ground water samples belong to very hard water (Sawyer McCarthy 1967). Hard water is an esthetic concern because of the unpleasant taste and because it decreased the ability of soap to produce scale in pipes and on plumbing fixtures.
Chemical constituents in groundwater
Calcium concentration ranged from 6 to 480 mg/l, with an average value of 78.2 mg/l. The maximum allowable limit of calcium concentration in groundwater is 200 mg/l as perWHO 2004 classification. In the comparison of ground water quality with the WHO standards, 92 % of the samples belong to the maximum allowable limit and the remaining 8 % of samples exceed the permissible limit (Table 3). Additionally, it is observed that the calcium concentrations are low compared to other cations. This lower concentration of calcium and sulfates may be due to the reaction of calcium with sulfates and subsequent precipitation. Magnesium concentration ranged from 9 to 194 mg/l, with an average value of 74.18 mg/ l. The allowable limit of magnesium ion concentration in groundwater is 150 mg/l as per the WHO 2004 standard. It exhibits that 92 % of the samples are represented by the maximum allowable limit and the remaining 8 % of the samples exceed the permissible limit. This is due to the dissolution of magnesium-bearing minerals in rocks, and also the sources are animal, domestic, and industrial wastes.
Potassium concentration varied between 1 and 215 mg/l, with an average value of 26.77 mg/l. The maximum allowable limit of potassium ion concentration in groundwater is 0.1 to10 mg/l, consistent with the WHO 2004 standards. The maximum allowable limit of the samples represented by 17 and 83 % of the samples exceeds the permissible limit.Figure 3a displays that the entire central portion, NW, and NE are observed as NP limit, whereas west, south, and NE fall within the permissible limit. Potassium concentrationwas very high in POM due to the dissolution of K-feldspars and clay minerals from the aquifer matrix (Lakshmanan et al. 2003). Sodium concentration ranged from 4 to 575 mg/l, with an average value of 146 mg/l.
The WHO standards exhibit that 79 % of the samples fallin the maximum allowable limit and the remaining 21 % samples exceed the permissible limit. This may be attributed to the weathering of rock-forming minerals like sodium plagioclase and halite and the influence of domestic and animal wastes. The NP limit of sodium was noted in some parts of NW, and small patches in the northern partand in other parts fall within the permissible limit (Fig. 3b). The high concentration of sodium may pose a risk to the persons suffering from cardiac, renal, and circulatory changes.
 Bicarbonate concentration ranged from 64 to 769 mg/l, with an average value of 403 mg/l. The allowable limit of drinking water uses by the WHO 2004 is 300 mg/l. Eightyonepercent of the samples are present within the permissible limit based on theWHO. The NP limit of HCO3 was observed in N-NW, N-NE, S-SE, and central region (Fig. 3c). The remaining parts are considered as permissible limit. This revealed that dissolution of minerals, like calcite and dolomite, and secondary sources can result from dissolution of CO2 gas likely formed by the anoxic biodegradation of organic matter that can be derived from industrial and domestic sewage, septic tanks, and buried waste in landfills (Cantor 1997; Jeong 2001; Zilberbrand et al. 2001). Chloride concentration varied from 7 to 167 mg/l, with an average value of 272 mg/l.
Bicarbonate concentration ranged from 64 to 769 mg/l, with an average value of 403 mg/l. The allowable limit of drinking water uses by the WHO 2004 is 300 mg/l. Eightyonepercent of the samples are present within the permissible limit based on theWHO. The NP limit of HCO3 was observed in N-NW, N-NE, S-SE, and central region (Fig. 3c). The remaining parts are considered as permissible limit. This revealed that dissolution of minerals, like calcite and dolomite, and secondary sources can result from dissolution of CO2 gas likely formed by the anoxic biodegradation of organic matter that can be derived from industrial and domestic sewage, septic tanks, and buried waste in landfills (Cantor 1997; Jeong 2001; Zilberbrand et al. 2001). Chloride concentration varied from 7 to 167 mg/l, with an average value of 272 mg/l. Maximum allowable limit of chloride ion concentration in groundwater is 600 mg/l. Eighty-nine percent of the samples belong to the maximum allowable limit and 11 % of the samples exceed the permissible limit. The maximum permissible limit of Cl is 600 mg/l that was observed toward the SWand SE regions, while NP limit was detected as small patches in the northwest region. Chlorite derived from weathering ofminerals like halite as well as domestic and fertilizer effluents (Loizidou and Kapetanios 1993).
Sulfate concentration ranged from 2 to 394 mg/l, with an average value of 57 mg/l. The maximum allowable limit of sulfate concentration on groundwater is 400 mg/l by theWHO2004 standard, and all the samples fall within the permissible limit. Nitrate and fluoride concentrations varied from 1 to 59 and 0.1 to 3 mg/l, respectively. Maximum allowable limit of nitrate (45 mg/l) and fluoride (1.5 mg/l) ion concentration in groundwater is represented by the WHO 2004. The WHO standards exhibit that 94%(NO3) and 70%(F) of the samples fall in the maximum allowable limit and 6 % (NO3) and 30 % (F) of the samples exceed the permissible limit. Nitrate derived from agricultural areas due to leaching processes from plant nutrients and nitrate fertilizers (Freeze and Cherry 1979; Madison and Brunett, 1985). In the case of fluoride, concentration in groundwater was not uniform.
This may be due to the occurrence and availability of fluorine-bearing mineral dissolution or anthropogenic activity. The most important fluoride-bearing minerals, apatite and fluorite or fluorapatite, are not commonly found in the granite unit in this area (Li et al. 2012), and water–rock interaction mainly by biotite in granite or possibly pegmatite probably plays an important role. Industrial facilities such as brick kilns and fertilizer are the reported possible sources of the fluoride from the surface.
Groundwater types and processes
Figure 4a displays that groundwater samples are categorizedas various chemical types on the Piper diagram (Piper 1944).Dominant water types are in the order of (CaHCO3)2 >CaMgCl > mixed CaNaHCO3 > NaCl. However, most ofthe samples are bunched in (CaHCO3)2 and CaMgCl segments. CaMgCl and (CaHCO3)2 type of water is indicatedas the mixture with high-salinity water caused by surface contaminationsources such as irrigation return flow, domestic wastewater, and septic tank effluents, with existing water followed by ion exchange reactions. However, mixed CaNaHCO3 and NaCl water types are represented by mineraldissolution and recharge of freshwater.
 The Gibbs diagram (Gibbs 1970) (Fig. 4b) exhibited that 35 % of the samples are influenced by chemical weathering and dissolution of rock-forming minerals. However, 65 % of the samples were in evaporation dominance zone. This may be attributed to salinity variation of Na and Cl and TDS. Agricultural activities may significantly influence the evaporation processes (Ravikumar et al. 2011; Sahu and Sikdar 2008). It is observed that the water chemistry of the Dindugal area occupies the field categorized as rock dominance as well as evaporation–crystallization dominance zone. This may be due to the weathering of granitic gneisses and the leaching of evaporated and crystallized ions from the top soils of the irrigation fields and improperly treated industrial effluent ponds. This clearly suggested that evaporation processes mainly controlled the groundwater chemistry of this region.
The Gibbs diagram (Gibbs 1970) (Fig. 4b) exhibited that 35 % of the samples are influenced by chemical weathering and dissolution of rock-forming minerals. However, 65 % of the samples were in evaporation dominance zone. This may be attributed to salinity variation of Na and Cl and TDS. Agricultural activities may significantly influence the evaporation processes (Ravikumar et al. 2011; Sahu and Sikdar 2008). It is observed that the water chemistry of the Dindugal area occupies the field categorized as rock dominance as well as evaporation–crystallization dominance zone. This may be due to the weathering of granitic gneisses and the leaching of evaporated and crystallized ions from the top soils of the irrigation fields and improperly treated industrial effluent ponds. This clearly suggested that evaporation processes mainly controlled the groundwater chemistry of this region.Identification of groundwater sources by WQI and statistical approach
Spatial distribution of WQI map
The application of the WQI was a good means to evaluate absolute water quality as it relates to the national water quality guidelines. This study was used based on the BIS (1998) guidelines that were applied to assess the variation of spatial and temporal changes in water quality. WQI exhibits that 17, 24, 27, 15, and 17 % of the groundwatersamples are in and that water is unsuitable for drinking purposes, respectively (Table 4). The GIS spatial map of WQI values suggested that a major part of the study areafalls in the poor category. This map shows the N–NS and NW parts. This is may be attributed to irrigation and domestic effluents (Fig. 5).
PCA
PCA suggested that HCO3 and K correlated with Ca, Mg, Na, and K. Ca and Mg are significantly influenced by seawater intrusion (Table 5). Na and K derived from agricultural activities (Li et al. 2012; Subba Rao 1998; Tizro and Voudouris2008). EC were correlated with Mg and F ions. The positive factor loadings with pH and F ions are represented by dissolution of fluoride-bearing minerals in the aquifer system. Scree plot was used to classify the number of PCs taken to understand the underlying data structure (Liu et al. 2008). It is used to specify the point of inflection on the curve. From the scree plot, a drop in the slope is renowned after the fourth eigenvaluewhich indicates the domination of four factors in the water chemistry. Hence, a total of four factors were assumed for this study, which explained about 51 % of the total variance.

Factor analysis
Four kinds of factors were identified which were controlling the groundwater chemistry of this region. The eigenvalues of these four factors were shown in cumulative percentage of 78.80 of variance (Table 5). Factor 1 consists of 47.36 % variance in the data set, and variables of EC, HCO3, and Cl derived from carbonate weathering and reverse ion exchange processes.A 13.01%variance with high loading for Na and K in factor 2 may be attributed by silicate (albite and orthoclase) weathering processes. Factor 3 exhibits 10.10 % of variance and loading variables like K, CO3, and PO4 which came from agricultural wastes. Factor 4 suggests 8.32 % of variance with high positive loadings for F. It indicates the weathering of fluoride minerals. It is concluded that various processes, such as weathering, ion exchange, and mineral dissolution, as well as anthropogenic effects, controlled the groundwater chemistryof this region.
Conclusion
The results of this research show that the hydrogeochemical study, coupled with multivariate statistical analyses, can elucidate the degrees and sources of groundwater contamination. Na+, K, HCO3, Cl, and F ions exceeded the limit of drinkingwater standard (WHO 2004). (CaHCO3)2 > CaCl2, MgCl2 > mixed (CaHCO3)2, and NaHCO3 > NaCl types are predominant in this region. The Gibbs diagram revealed that most of the groundwater sample fell at rock dominance field, indicating the dissolution of minerals and other anthropogenic activities. The correlation coefficient between HCO3 with EC/TDS, Cl and EC/TDS, HCO3 and F, Na with EC/TDS, Ca with EC/TDS, Mg with EC/TDS, HCO3 with Cl, Na with Cl, Ca with Cl, and Ca with Na shows strong positive correlation.
Four factors, with 64 % of total variance, indicated the domination of silicate weathering, ion exchange, leaching, and anthropogenic effects. TheWQI maps suggested that small patches in NW, NE, S, and SE directions had very poor groundwater quality. Based on this research, the ion concentration of groundwater could be derived from both anthropogenic and natural sources. As a result, further research is necessary to understand the behavior of groundwater chemistry in this region.
Acknowledgments
The author s are thankful to Shri A.P.C.V.Chockalingam, Secretary, and Dr.C.Veerabahu, Principal, from V.O.C College, Tuticorin, for their support to carry out this study. Theauthors are also thankful to the anonymous reviewers who have provided their valuable suggestions to improve the manuscript.
References
Antony Ravindran A, Selvam S (2014) Coastal disaster damage and neotectonic subsidence study using 2D ERI technique in Dhanushkodi, Rameshwaram Island, Tamilnadu, India. Middle-East J Sci Res 19(8):1117–1122
APHA (1995) Standard methods for the examination of water and wastewater,19th edn. American Public Health Association, New York BIS (1998) Specifications for drinking water, New Delhi, Pp 171-178 Burrough PA, McDonnell RA (1998) Principles of geographical information systems Oxford: Oxford University Press, p. 333.
Cantor R (1997) Drinking water and cancer. Cancer Causes Control 8(3): 292–308
Chidambaram S, Vijayakumar V, Srinivasamoorthy K, Anandhan P, Prasanna MV, Vasudevan S (2007) A study on variation in ionic composition of aqueous system in different lithounits around Perambalur region, Tamil Nadu. J GeolSoc India 70(6):1061–1069 Davis SN, DeWiest RJ (1966) Hydrogeology. Wiley, Newyork. 463
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall, New Jersey, p. 56
Gibbs RJ (1970) Mechanisms controlling world’s water chemistry. Science 170:1088–1090
Handa BK (1969) Description and classification of media for hydrogeochemicalinvestigations. In Symp on Ground Water Studies in Arid and Semiarid Regions [M]. Roorkee. India
Hem JD (1991) A study and interpretation of the chemical characteristics of natural water, book 2254, 3rd edn. Scientific Publication, Jodhpur, p. 263
Irawan DE, Puradimaja DJ, Notosiswoyo S, Soemintadiredja P (2009) Hydrogeochemistry of volcanic hydrogeology based on cluster analysis of Mount Ciremai,West Java, Indonesia. J Hydrol 376:221–234
Jeong CH (2001) Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol 253:194–210
Karanth KR (1991) A text book of groundwater assessment, Development and management, Tata Mc Graw Hill Publishing Company Ltd, New Delhi. Pp659
Kazi TG, Arain MB, Jamali MK, Jalbani N, Afridi HI, Sarfraz RA, Baig JA, Shah AQ (2009) Assessment of water quality of polluted lakeusing multivariate statistical techniques: a case study. Ecotox Environ Safety 72:301–309
Lall U, Sharma A (1996) A nearest neighbor bootstrap for resampling hydrologic time series. Water Resour Res 32(3):679–693
Lakshmanan E, Kannan K, Senthil KumarM(2003) Major ion chemistry and identification of hydrogeochemical process of groundwater in part of Kancheepuram district, Tamilnadu, India. J Environ Geosci 10(4):157–166
Li JX, Wang YX, Xie XJ, Su CL (2012) Hierarchical cluster analysis of arsenic and fluoride enrichments in groundwater from the Datong basin, Northern China. J Geochem Explor 118:77–89
Liu CW, Jang CS, Chen CP, Lin CN, Lou KL (2008) Characterization of groundwater quality in Kinmen Island using multivariate analysis and geochemical modelling. Hydrol Process 22:376–338
Loizidou M, Kapetanios EG (1993) Effect of leachate from landfills on underground quality. Sci Total Environ 128:69–81. doi:10.1016/0048-9697(93)90180EMagesh NS, Chandrasekar N, Vetha Roy D (2011) Spatial analysis of trace element contamination in sediments of Tamiraparani estuary, southeast coast of India. Estuar Coast Shelf Sci 92:618–628
Magesh NS, Chandrasekar N (2013) Evaluation of spatial variations in groundwater quality by WQI and GIS technique: a case study of Virudunagar district, Tamil Nadu, India. Arab J Geosci 6:1883–1898
Madison RJ, Brunett JO (1985) Overview of the occurrence of nitrate in ground water in the United States. In: U.S. Geological Survey, National Water Summary 1984—hydrologic events, selected water-quality trends, and ground-water resources. U.S. Geol Surv Water-Suppl Pap 2275:93–105
Mondal NC, Saxena VK, Singh VS (2005) Assessment of groundwater pollution due to tanneries in and around Dindigul, Tamilnadu, India. Environ Geol 48:149–157
Mondal NC, Singh VP, Singh VS, Saxena VK (2010) Determining the interaction between groundwater and saline water through groundwater major ions chemistry’. J Hydrol 388(1–2):100–111
PWD (Public Works Department) (2000) Groundwater perspectives, a profile of Dindigul district. PWD Report, Tamilnadu, p. 102
Pandit AK (2002) Topical evolution of lakes in Kashmir Himalaya. In: Pandit AK (ed) Natural resources of Western Himalaya. Valley Book House, Srinagar J&K, pp. 213–242
Paul Basker J (2000) Tanneries in Dindigul district, dossier on tannery pollution in Tamilnadu, compiled and produced by Peace Trust, pp 195–210.
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis trans. Am Geophys Union 25:914–923
Ravikumar P, Somashekar RK, Angami M (2011) Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State, India^. Environ Monit Assess 173(1–4):459–487
Ravikumar P, Mohammad Aneesul M, Somashekar RK (2013) Water quality index to determine the surface water quality of Sankey tank and Mallathahalli Lake, Bangalore urban district, Karnataka. India Appl Water Sci. doi:10.1007/s13201-013-0077-2
Sahu P, Sikdar PK (2008) Hydrochemical framework of the aquifer in and around East Kolkata wetlands,West Bengal. India Environ Geol 55: 823–835
Sarbu C, Pop HF (2005) Principal component analysis versus fuzzy principalcomponent analysis, a case study: the quality of Danube water (1985–1996). Talanta 6:1215–1220
Sawyer C, McCarthy P (1967) Chemical and sanitary engineering, 2nd edn. McGraw-Hill, New York
Subba Rao N (1998) Groundwater quality in crystalline terrain of Guntur district, Andhra Pradesh. India Visakha Sci J 2:51–54
Selvam S (2012) Use of remote sensing and GIS techniques for land use and land cover mapping of Tuticorin Coast, Tamilnadu. Univers J Environ Res Technol 2(4):233–241
Selvam S (2014a) Irrigational feasibility of groundwater and evaluation of hydrochemistry facies in the SIPCOT industrial area, South Tamilnadu. India: A GIS Approach. Water Qual Expo Health. Doi: 10.1007/s12403-014-0146-2
Selvam S (2015) A preliminary investigation of lithogenic and anthropogenic influence over fluoride ion chemistry in the groundwater of the southern coastal city, Tamilnadu, India. Environ Monit Assess 187:106. doi:10.1007/s10661-015-4326-8
Selvam S, Iruthaya Jeba Dhana Mala R, Muthukakshmi V (2013a) A hydrochemical analysis and evaluation of groundwater quality index in Thoothukudi district, Tamilnadu, South India. Int J Adv Eng Appl 2(3):25–37
Selvam S, Manimaran G, Sivasubramanian P (2013b) Hydrochemical characteristics and GIS-based assessment of groundwater quality in the coastal aquifers of Tuticorin corporation, Tamilnadu, India. Appl Water Sci 3:145–159
Selvam S,Manimaran G, Sivasubramanian P (2013c) Cumulative effects of septic system disposal and evolution of nitrate contamination impact on coastal groundwater in Tuticorin, South Tamilnadu, India. Res J Pharm Biol Chem Sci 4(4):1207–1218
Selvam S, Manimaran G, Sivasubramanian P, Balasubramanian N, Seshunarayana T (2014a) GIS-based evaluation of water quality index of groundwater resources around Tuticorin coastal city, South India. Environ Earth Sci 71:2847–2867
Selvam S, Antony Ravindaran A, Rajamanickam M, Sridharan M (2014b) Microbial contamination in the sediments and groundwater of Tuticorin Corporation, South India using GIS. Int J Pharm Pharm Sci 6(4):337–340
Selvam S, Manimaran G, Sivasubramanian P, Seshunarayana T (2014c) Geoenvironmental resource assessment using remote sensing and GIS: a case study from southern coastal region. Res J Recent Sci 3(1):108–115
Selvam S,Magesh NS, Chidambaram S, Rajamanickam M, Sashikkumar MC (2014d) A GIS based identification of groundwater recharge potential zones using RS and IF technique: a case study in Ottapidaram taluk Tuticorin district. Tamil Nadu Environ EarthSci. doi:10.1007/s12665-014-3664-0
Selvam S (2014b) Irrigational feasibility of groundwater and evaluation of hydrochemistry facies in the SIPCOT industrial area South Tamilnadu. India: A GIS Approach Water Qual Expo Health. Doi: 10.1007/s12403-014-0146-2
Selvam S, Magesh NS, Sivasubramanian P, Prince Soundranayagam J, Manimaran G, Seshunarayana T (2014e) Deciphering of groundwater potential zones in Tuticorin, Tamil Nadu, using remote sensing and GIS techniques. J Geol Soci of India 84:597–608
Selvam S,Magesh NS, Chidambaram S, Rajamanickam M, Sashikkumar MC (2015a) A GIS based identification of groundwater recharge potential zones using RS and IF technique: a case study in Ottapidaram taluk, Tuticorin district Tamil Nadu. Environ EarthSci 73:3785–3799
Selvam S, Venkatramanan S, Singaraja C (2015b) A GIS-based assessment of water quality pollution indices for heavy metal contamination in Tuticorin Corporation Tamilnadu. India Arab J Geosci. Doi: 10.1007/s12517-015-1968-3
Selvam S, Dar FA, Magesh NS, Singaraja C, Venkatramanan S, Chung SY (2015c) Application of remote sensing and GIS for delineating groundwater recharge potential zones of Kovilpatti municipality. Tamil Nadu Using IF Technique Earth Sci Inform. doi:10.1007/s12145-015-0242-2
Singaraja C, Chidambaram S, Srinivasamoorthy K, Anandhan P, Selvam S (2015) A study on assessment of credible sources of heavy metal pollution vulnerability in groundwater of thoothukudi districts Tamilnadu. India Water Qual Expo Health. doi:10.1007/s12403-015-0162-x
Srinivasamoorthy K, Nanthakumar C, Vasanthavigar M, Vijayaraghavan K, Rajivgandhi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2011) Groundwater quality assessment from a hard rock terrain, Salem district of Tamil Nadu, India. Arab J Geosci 4: 91–102. doi:10.1007/s12517-009-0076-7
Tizro TA, Voudouris K (2008) Groundwater quality in the semi-arid region of the Chahardouly Basin, West Iran. Hydrol Process 22(16): 3066–3078
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV (2012) Evaluation of groundwater suitability for domestic, irrigational, and industrial purposes: a case study from Thirumanimuttar River Basin, Tamil Nadu, India. Environ Monitor Assess 184(1):405–420
Venkatramanan S, Ramkumar T, Anithamary I (2012) A statistical approachon hydrogeochemistry of groundwater in Muthupet coastal region, Tamil Nadu, India. Carpathian J Earth Environ Sci 7:47–54
Venkatramanan S, Chung SY, Ramkumar T, Gnanachandrasamy G, Vasudevan S (2013) A multivariate statistical approaches on physicochemical characteristics of groundwater in and around Nagapatttinam district, Cauvery deltaic region of Tamil Nadu,India. Earth Sci Res J 17:97–103
Venkatramanan S, Chung SY, Kim TH, Prasanna MV, Hamm SY (2014) Assessment and distribution of metals contamination in groundwater: a case study of Busan city, Korea. Water Qual Expo Health 7: 219–225. doi:10.1007/s12403-014-0142-6
Venkatramanan S, Chung SY, Ramkumar T, Rajesh R, Gnanachandrasamy G (2015) Assessment of groundwater quality using GIS and CCME WQI techniques: a case study of Thiruthuraipoondi city in Cauvery deltaic region, Tamil Nadu, India. Desalination and Water Treatment, P 1-16.doi:10.1080/19443994.2015.1048740.
WHO (2004) Guidelines for drinking water quality, vol 1. Recommendations, 3rd edn. WHO, Geneva
Zilberbrand M, Rosenthal E, Shachnai E (2001) Impact of urbanization on hydrochemical evolution of groundwater and on unsaturatedzone gas composition in the coastal city of Tel Aviv, Israel. J Contam Hydrol 50:175–208